Choosing CNP: The Science Behind Human Growth
June 20, 2024
Dan Wendt has many memories from his 26 years working at BioMarin, but one day in particular still sticks out in his mind.
It was a morning in 2007 when a group of researchers came to BioMarin to present their findings from a study on the interaction of fibroblast growth factor receptor (FGFR) and C-type natriuretic peptide (CNP) signaling in regulating growth in preclinical models.
“The research was very compelling, and it quickly became clear to us that there was evidence starting to emerge for CNP as a master regulator of growth-related development,” recalls Dan, Director, CNP Franchise Lead, Musculoskeletal Research at BioMarin. “We immediately began work on a research program focused on skeletal conditions and about a year later had the first derivative of what would become our investigational treatment for the most common cause of genetic short stature, achondroplasia.”
Since that pivotal day in 2007, Dan and BioMarin’s teams of researchers have led major scientific breakthroughs that have helped contribute to the scientific community’s understanding of skeletal conditions. The company’s approach – using a CNP analog to tap into the natural processes that determine height – has been rigorously tested in more than 10 clinical trials and continues to be evaluated in real-world settings with more than 3,100 individuals receiving treatment to date.
“At BioMarin, we’ve followed the science with CNP as the optimal target for our research into genetic skeletal conditions,” says Marcia Kayath, BioMarin’s Senior Vice President and Head of Global Medical Affairs, who began her career as a physician and endocrinologist. “We’ve rigorously validated this approach in clinical studies — resulting in approximately 4,500 patient-years of data in clinical trials and real-world usage, with peer-reviewed data featured in more than 50 publications and presentations — and we’re just getting started.”
The Genetics of Human Height
Height is one of the traits most clearly linked to genetics. In studies of thousands of genomes, one signal in particular stood out as a master regulator of height: CNP.
“Quite simply, these large-scale genomic studies revealed that when CNP is overexpressed, it can result in tall stature, while loss of CNP signaling can result in short-stature conditions,” explains Chris Bauer, Associate Director, Statistical Genetics at BioMarin. “This bidirectional impact on height has been confirmed in laboratory studies that evaluated the effects of either increasing or blocking CNP signaling.”
Another key finding from early genomic studies was the connection between height and the FGFR3 gene. Using DNA sequencing, researchers were able to identify alterations in the FGFR3 gene as the root cause of achondroplasia, the most common form of genetic short stature. The genetic change produces an altered form of the FGFR3 protein that continuously sends a signal to slow down bone growth, resulting in the physical manifestations of skeletal dysplasias, including short stature.
The genetic studies revealed one other crucial insight: changes in CNP and FGFR3 levels have fundamentally different impacts on height, with CNP signaling appearing to be highly targeted to impact skeletal growth.
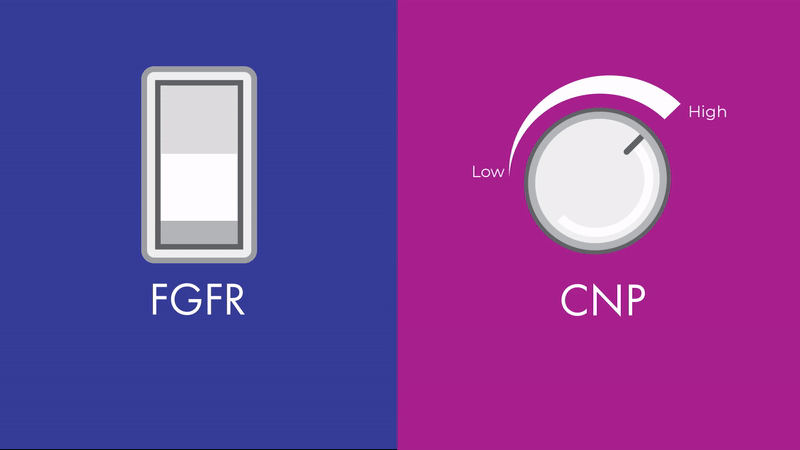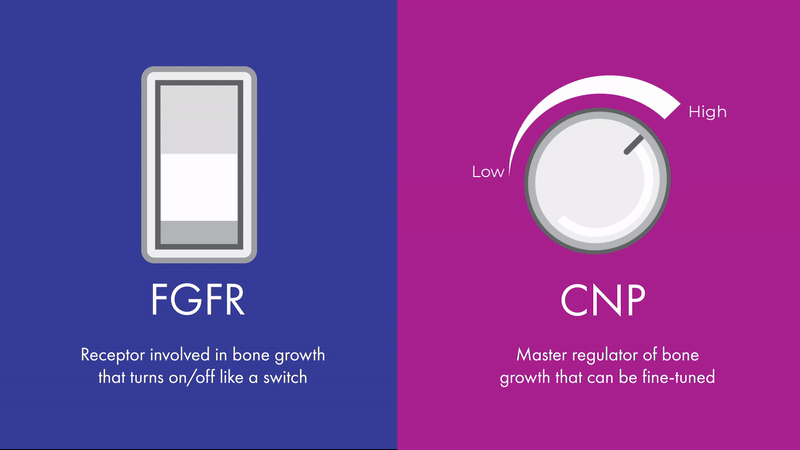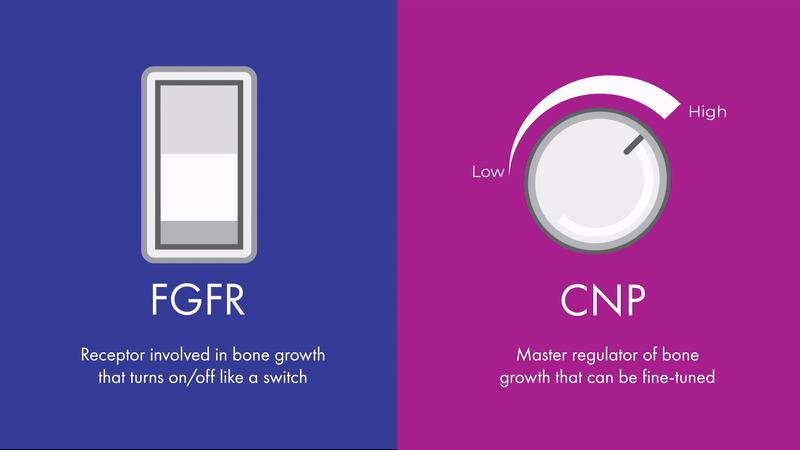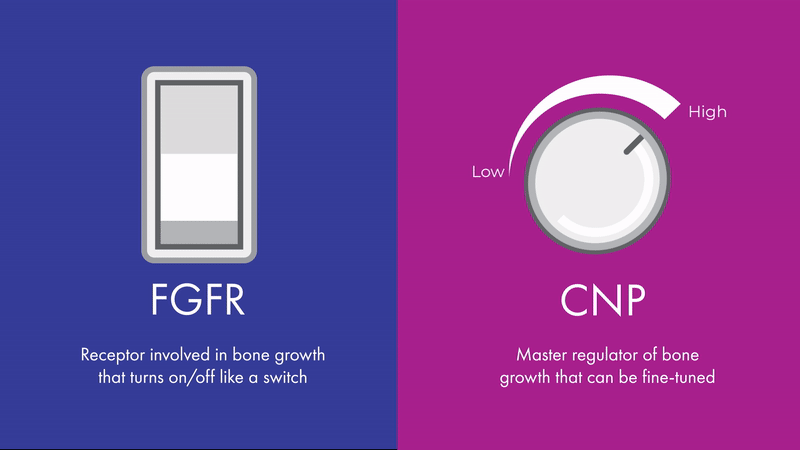
“The effects of CNP signaling on height are bidirectional and fine-tunable, like a dimmer light switch,” says Chris. “While FGFR3 is also involved in bone growth, its effects are more like an on-off switch, which can’t be fine-tuned in the same way.”
This led BioMarin scientists to wonder if these insights could be leveraged to develop a more nuanced, adjustable approach that could be evaluated in skeletal conditions.

Members of BioMarin’s Musculoskeletal research team have been investigating the role of CNP for years.
Navigating Pathways to Find the Ideal Target
When Dan attended that 2007 research presentation on the interaction between CNP and FGFR3, the exact mechanisms of the molecular interactions were not fully understood.
“FGFR3 signaling can activate a veritable alphabet soup of different signaling pathways, including MAPK/ERK, JAK/STAT, and mTOR/AKT,” he says. “But our teams at BioMarin and other researchers in the field eventually identified MAPK/ERK as the overactive pathway to target – and this is the pathway where CNP operates.”
Unlike FGFR3, CNP signaling can be fine-tuned, making it a promising therapeutic target across multiple skeletal conditions. Contrary to diseases such as cancer, where malignant growth needs to be turned off entirely, skeletal conditions may benefit from a more nuanced approach.
“Based on the clear scientific evidence, we were convinced that if we developed a CNP analog, it could fundamentally change the trajectory of how we approach therapeutic options for skeletal conditions,” says Dan. “The great thing is that the CNP analog leverages one of the body’s own natural processes to impact height. Plus, this approach bypasses FGFR3, so it could have broad applicability not limited to conditions where changes in FGFR3 are present.”
From Scientific Insight to Clinical Reality
Decades of scientific research at BioMarin not only validated the role of CNP, but also helped change the clinical landscape for families impacted by skeletal conditions.
This deepened understanding of CNP may support the development of medicines for several skeletal conditions. BioMarin continues to explore the potential for this approach to impact people living with hypochondroplasia, idiopathic short stature (ISS) and multiple genetic short-stature conditions, including Turner syndrome, SHOX deficiency and Noonan syndrome.
“We’re excited to be moving forward with the broadest clinical development program in genetic skeletal conditions, building on the robust body of clinical evidence we’ve already generated,” says Marcia. “Especially for hypochondroplasia and other skeletal conditions, where there are no currently approved medicines available to treat the underlying causes, we’re moving rapidly to explore how our approach with CNP could provide families with better outcomes.”



