What is VOXZOGO®▼
▼This medicinal product is subject to additional monitoring in Australia. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse events at www.tga.gov.au/reporting-problems.
VOXZOGO® (vosoritide) is indicated for the treatment of achondroplasia in patients 2 years of age and older whose epiphyses are not closed. The diagnosis of achondroplasia should be confirmed by appropriate genetic testing.
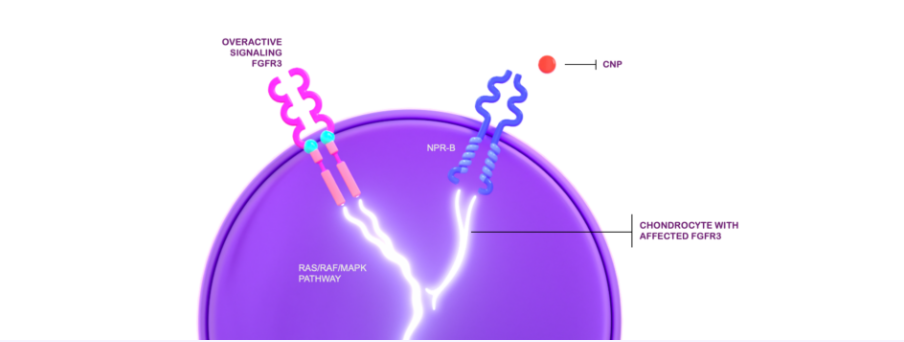
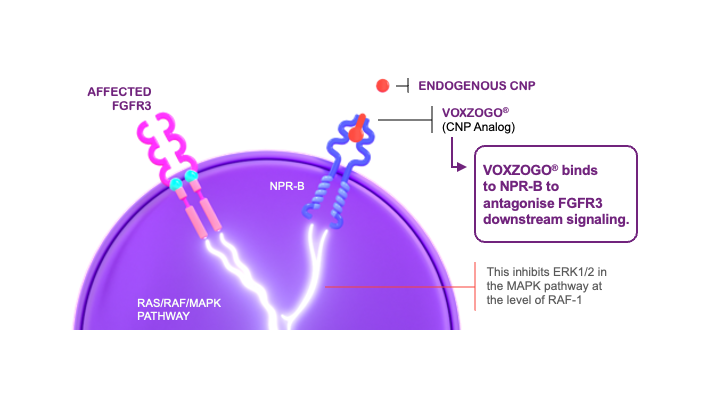
In achondroplasia, the endogenous C-type natriuretic peptide (CNP) cannot adequately regulate the overactive fibroblast growth factor receptor 3 (FGFR3) signaling.1,2
This affects the proliferation and terminal differentiation of growth plate chondrocytes, resulting in impaired endochondral bone growth and linear growth.3 VOXZOGO®, a biological CNP analog, acts as a positive regulator of endochondral bone growth, targeting overactive FGFR3 signaling and promoting chondrocyte proliferation and differentiation.