The safety and efficacy of VOXZOGO® (vosoritide) were evaluated in patients with achondroplasia aged 5 to 15 years1,2
Statistically significant improvements in annualised growth velocity (AGV) were seen in patients aged 5 to 15 years1
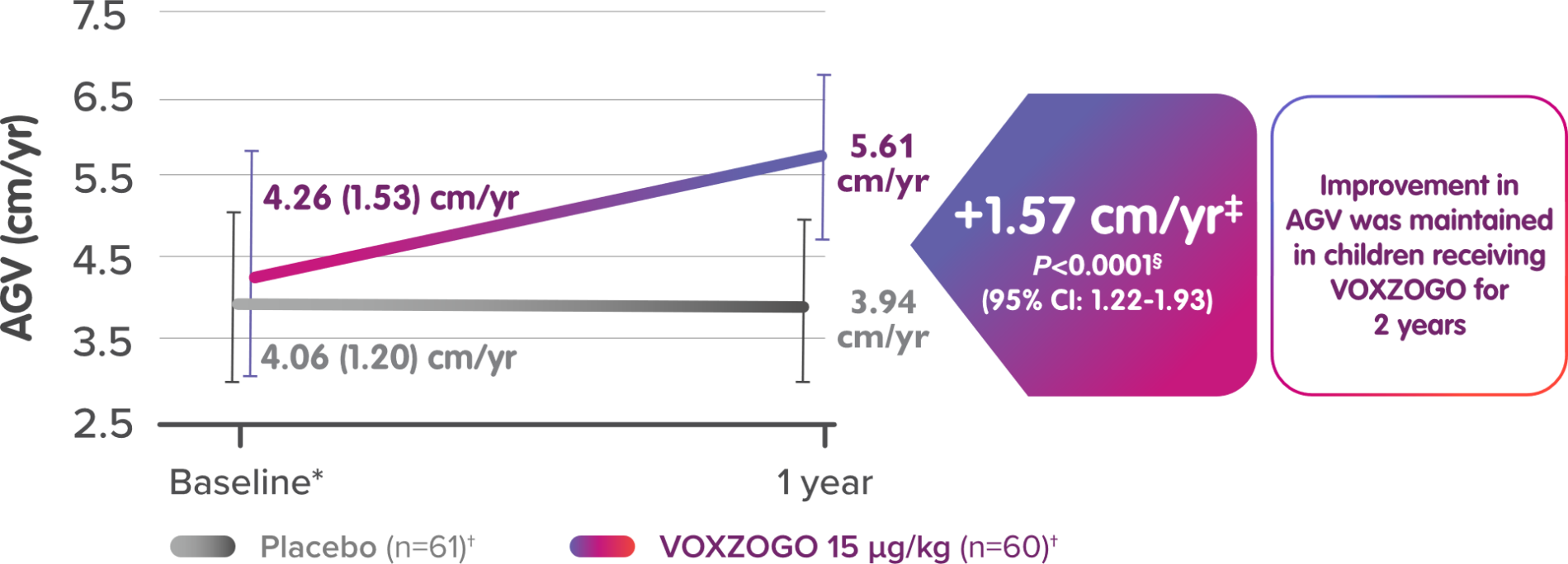
Mean (SD) AGV results over baseline from a placebo-controlled clinical trial1-3
SD, standard deviation.
*LS means were estimated from the ANCOVA (analysis of covariance) model, which included treatment, stratum defined by sex and Tanner stage, baseline age, baseline AGV, and baseline height Z-score.
†All randomised subjects. Two patients in the VOXZOGO group discontinued from the study before Week 52. The values for these 2 patients were imputed assuming baseline growth rate for the period with missing data.
‡Baseline AGV was based on standing height at least 6 months prior to enrollment into the study.
§Two-sided P value.
